What is Ozone?
Ozone is a three-atom oxygen molecule that reacts rapidly and is highly unstable due to its unstable structure. It reacts with an oxygen molecule and a new born oxygen atom, which reacts quickly.For this reason, ozone is one of the most powerful sterilizing agents (bacteria, viruses and odors) in the world. Ozone production in nature is done in two ways: ultraviolet and lightning. The ozone layer is created by the sun’s ultraviolet rays. The basis of an ozone generator can also be UV light or high voltage. In the UV method, ozone is produced by exposing the UV lamps to the oxygen entering the reactor. This is the most basic method of producing ozone. In the electric discharge method, two high voltage difference electrodes are inserted. Oxygen passes between these two electrodes and between these two electrodes the arc is formed and the oxygen molecules are converted to ozone.

Schematic form of operation of the ozone generating reactor
Electric discharge method is superior to the ultraviolet method in two ways: First, in the ultraviolet way, UV lamps need to be replaced periodically, and this will impose a huge cost on the consumer. In addition, the advantage of ozone generators is the reduction of current costs and no need for operator. The second major advantage of the electric discharge method, which is more important than the first reason, is the ozone to oxygen ratio at the ozone generator outlet. The higher the purity of ozone at the output of the device, the greater the solubility of ozone in the water. For example, by increasing the purity from 1% to 10%, the solubility of ozone in water is six times higher.
How does ozone work?
Ozone is based on oxidation and due to its instability has strong oxidizing properties. Because the new born oxygen atom is interested in reducing its oxidation number by two units, it oxidizes other molecules. Ozone can oxidize odor, color, opacity and microorganisms such as viruses, fungi and bacteria and this ozone has been used as a material for treatment in various fields such as industrial treatment plants, drinking water packaging, swimming pools, poultry, animal husbandry and textile. The most important benefit of ozone is its cleanliness, as it produces almost no byproducts. Ozone, on the other hand, has a strong and recognizable odor, so its low concentrations can be understood, making it safe to operate with ozone gas.
What is the correct density of ozone?
The proper amount of ozone is when all the produced ozone is consumed and the residual ozone density is below the permitted level. The ozone density level for different organisms varies. Ozone levels will also vary in aquaculture and fisheries. The reaction time and the effect of ozone are also effective in determining the allowable limit. For example, 0.02ppm is the ozone limit for fish fidding that is in contact with ozone gas for 2 to 5 hours.
Ozone is 0.12 ppm for swimming for 2 hours swimming and 0.2 ppm is the Density that should be discharged to the environment.
How to determine the level of ozone?
There are many measurement tools available to measure the amount of ozone in the weather that can measure and even control its amount in ppm. The ozone meter device is essential for the ozone maker companies and if ozone packages are installed in fish farms, removing ozone remnants will be one of the most important things to pay attention to.
How long is ozone live?
Ozone is eliminated as soon as it is produced in ozone maker and released into the environment through processes such as reaction with bacteria, reaction with organic and inorganic substances. In addition, ozone has a half-life, meaning that any amount of ozone remaining after a certain time during the natural decay process converts to its initial half-oxygen level.
What is the half-life of ozone?
Due to the low half-life of ozone (about 15-30 minutes in water), ozone must be produced at the site in question for water purification as it disintegrates immediately after production. However, even its half-life in practice is less than that and depends on factors such as temperature, PH, contamination rate and ozone concentration.
Is Ozone Harmful? What are its effects?
Ozone at high density is harmful to human respiration, and institutions have stated that it is above the threshold level of ozone density, which means that prior to reaching the permissible density, ozone will be detectable by odor. When exposed to high density of ozone, they may feel dryness in the throat and mouth, cough, headache, or chest cramps. In addition to prevention and control, for example, where large-scale ozone generators are used, devices for the destruction of excess ozone can also be predicted and deployed.In fish farms, if water-soluble ozone is above 0.03ppm, the mucus of the fish’s body will be lost and subsequently burned in the gill area, and the fish will be killed due to loss of gill radius and respiratory disturbance.
How does ozone react with bacteria?
Most disinfectants such as chlorine, percidine, formalin, etc. To destroy the bacteria, must penetrate the cell nucleus and destroy it by destroying the cell nucleus.This will increase the strength of the reaction and the reaction time of these substances. But ozone disintegrates by destroying the cell surface and its selective membrane. As a result, it responds much faster and has more power.
The following is described together with pictures:
|
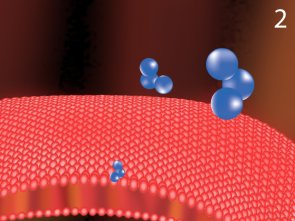
A healthy bacterial cell
|
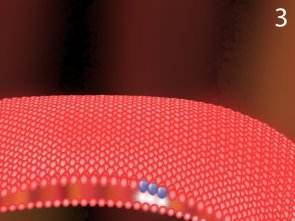
Ozone in contact with the bacterial cell wall
|
After ozone molecules contact the cell wall, it actually creates a small gap in the cell wall.
|
|
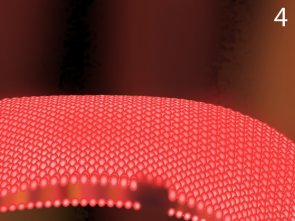
This gap can damage the bacteria. And if the ozone injection is continued, the bacteria will lose their shape.
|
After thousands of ozone collisions, the bacteria are unable to maintain their shape and die.
|
Eventually cell death
|
What microorganisms are killed by ozone?
PROTOZOA
Paramecium
Nematode eggs
Chlorella vulgaris Algae
All Pathogenic and Non-pathogenic forms of Protozoa
FUNGUS & MOLD SPORES
Aspergillus candidus
Aspergillus flavus yellowish-green
Aspergillus glaucus bluish-green
Aspergillus niger black
Aspergillus terreus, saitoi & oryzac
Botrytis allii
Colletotrichum lagenarium
Fusarium oxysporum
Grotrichum
Mucor recomosus A & B white-gray
Mucor piriformis
Oospora lactis white
Penicillium cyclopium
P. chrysogenum & citrinum
Penicillium digitatum olive)
Penicillium glaucum
Penicillium expansum olive
Penicillium egyptiacum
Penicillium roqueforti green
Rhizopus nigricans black
Rhizopus stolonifer
BACTERIA
Achromobacter butyri NCI-9404
Aeromonas harveyi NC-2
Aeromonas salmonicida NC-1102
Bacillus anthracis
Bacillus cereus
B. coagulans
Bacillus globigii
Bacillus licheniformis
Bacillus megatherium sp.
Bacillus paratyphosus
B. prodigiosus
Bacillus subtilis
B. stearothermophilus
Clostridium botulinum
C. sporogenes
Clostridium tetoni
Cryptosporidium
Coliphage
Corynebacterium diphthriae
Eberthella typhosa
Endamoeba histolica
Escherichia coli
Escherichia coli
Flavorbacterium SP A-3
Leptospira canicola
Listeria (ozone?)
Micrococcus candidus
Micrococcus caseolyticus KM-15
Micrococcus spharaeroides
Mycobacterium leprae
Mycobacterium tuberculosis
Neisseria catarrhalis
Phytomonas tumefaciens
Proteus vulgaris
Pseudomonas aeruginosa
Pseudomonas
fluorscens (bioflims)
Pseudomonas putida
Salmonella choleraesuis
Salmonella enteritidis
Salmonella typhimurium
Salmonella typhosa
Salmonella paratyphi
Sarcina lutea
Seratia marcescens
Shigella dysenteriae
YEAST
Baker’s yeast
Candia albicans- all forms
Common yeast cake
saccharomyces cerevisiae
saccharomyces ellipsoideus
saccharomyces sp.
VIRUS
AIDS
Adenovirus type 7a
Bacteriophage E.coli
Coxackie A9, B3, & B5
Cryptosporidium
Echovirus 1, 5, 12, &29
Encephalomyocarditis
Hepatitis A
GD V11 Virus
Onfectious hepatitis
Influenza
Legionella pneumophila
Polio virus (Poliomyelitus) 1, 2 & 3
Rotavirus
Tobacco mosaic
Vesicular Stomatitis
Rhizoctonia solani
Rhizopus stolonifera
Sclerotium rolfsii
Sclerotinia sclerotiorum
FUNGAL PATHONGENS
Alternaria solani
Botrytis cinerea
Fusarium oxysporum
Monilinia fruiticola
Monilinia laxa
Pythium ultimum
Phytophthora erythroseptica
Phytophthora parasitica
Shigella flexnaria
Shigella paradysenteriae
Spirllum rubrum
Staphylococcus albus
Staphylococcus aureus
Streptococcus ‘C’
Streptococcus faecalis
Streptococcus hemolyticus
Streptococcus lactis
Streptococcus salivarius
Streptococcus viridans
Torula rubra
Vibrio alginolyticus & angwillarum
Vibrio clolarae
Vibrio comma
Virrio ichthyodermis NC-407
V. parahaemolyticus
CYSTS
Cryptosporidium parvum
Giardia lamblia
Giardia
ALGAE
hlorella vulgaris
Thamnidium
Trichoderma viride
Verticillium albo-atrum
Verticillium dahliae
How to install an ozone system:
The equipment described below is used to install the ozone system.
- Ozone Maker
This device converts oxygen to ozone by electrical discharge technology. Here are some features of the ozone generator:
- The use of electric discharge phenomenon in the production of ozone
- Permanent industrial and semi-industrial work
- Equipped with water or air cooling system
- All-steel equipment and parts to prevent corrosion and high cleanability for use in sterile environments
- Use of ozone-resistant components in machine manufacturing
- Equipped with internal sensor to control the presence or absence of ozone
- 220V Single Phase Input Power 50-60 Hz
- Ozone output control from 10% to 100%
- Customizable to work in specific hours and change ozone output
- With digital board and digital programming capability
- Ability to connect to the control unit of the device user unit (eg control room)
- Oxygen Maker
The raw material for ozone production is oxygen. In ambient air there is 21% oxygen and the remaining 79% is nitrogen and other gases. An oxygen generator is used to increase the efficiency of the device. Oxygen generator separates oxygen from nitrogen and other gases in the air during a physical process using a zeolite tank similar to gas chromatography. The oxygen purity at the oxygenator output is more than 90%.
- Venturi and ozone injection equipment
Injecting ozone into the air or into the water requires an injector such as a venturi and a static mixer. Venturi’s work is based on reducing the pressure and suction of gas into the water. Ozone injection fittings also use one-way stainless steel valves to prevent water from returning to the device and other equipment that is not important.
Schematic form of water installation system
Ozone transfer units that have a continuous liquid phase (that is, units that disperse gas bubbles in a liquid), such as cones and tubes, suckers, bubble dispersers, etc., both transmit ozone and contact time. they prepare.
Cones for better mass transfer of ozone gas
The use of Ozone in Aquaculture
A: Pathogenic microorganisms and disease prevention
Removal of various microorganisms, including bacteria, spores, fungi, viruses, yeast and algae. Also, using ozone gas can be effective in controlling and preventing a variety of diseases.
The amount of ozone needed to eliminate any pathogen will be different, for example:
The amount of ozone needed to eliminate E.coli is 0.25ppm in 2 minutes and the amount of ozone injectable gas to be eliminated is 0.2ppm in 30 seconds. It will also take longer and longer to inject ozone to eliminate viruses.
B: Composition of iron and manganese and other minerals
Ozone converts iron and manganese from soluble to insoluble, in other words, ferrous (Fe2 +) to ferric ion (Fe3 +), which is highly insoluble in ferric hydroxide. After removal of heavy metals by ozone, proper filtration to remove insoluble heavy metals after oxidation should be performed.
The presence of this type of metal in the water not only reduces its quality but also precipitates and masses the ducts and valves and other metals such as manganese, cadmium, quaternary chromium, mercury, cobalt, copper, lead, arsenic, nickel and zinc can be refined with the same method.The reaction of hydrogen sulfide with ozone is also certain and ozone can supply its permissible density of 0.003 ppm for fish farming.In general, ozone promotes the oxidation of minerals to higher degrees of oxidation and stabilizes them.
C: Reduce ammonia and nitrite
Nitrogenous compounds, and especially ammonia (NH3) in aquaculture water, have a significant impact on reducing the quality of production and causing significant losses in fish. Ozone is capable of reacting with these compounds during the de-polymerization process and converts them to less toxic and stabilized compounds. The reaction of ozone with ammonia is as follows:
NH3+3O3 NO–2+3O2+H++H2O
NO–2+ O3 NO–3+O2
As it is known, one ammonia molecule with four ozone molecules is converted to nitrite and nitrate after reaction. Meanwhile, the fish tolerance threshold for ammonia is 0.03ppm, for nitrite 0.15ppm and for nitrate 3.00ppm.
In our studies, the tendency of ozone response to ammonia was higher than the pathogenic factors.
Also, The reaction of ozone to ammonia at high temperatures and at higher pHs will also be faster and more noticeable. As a result, the effect of ozonation in hydrothermal fish pools is evident and undeniable.
In research, the rate of reaction of ozone with ammonia is increasingly increased with the involvement of hydrogen peroxide (OH-), which can be combined with ozone and hydrogen peroxide injection systems at concentrations of more than one ppm.
D: Reduce water turbidity
The suspended particles below 50 microns that cause water turbidity can be oxidized by ozone gas and create a suitable environment for fish growth. Appropriate ozone levels are recommended for removal of such suspensions in feedlots and breeding farms with high densities of 15 ppm and in hatchery and nursery units of 3 ppm.
In addition, be sure to contact the experts at the FardFishery Department for ozone injections and get expert opinions on this, lest you suffer irreparable casualties.
E: Prevent algae growth
One of the most noticeable problems in the pools is the drainage and periodic cleaning of the floors and walls. The presence of algae and its clearance from pools has always been a challenge. Generally, algae are of primary origin, eliminating ozone or egg algae, delaying or preventing algae growth significantly.
Algae are one of the most important consumers of soluble oxygen at night. Therefore, the removal of algae will help preserve water-soluble oxygen and prevent PHC changes or fluctuations around the clock.
F: No change in water pH
Ozone has no effect on PH, but high pH of water has a favorable effect on ozone reactivity.
G: Increased dissolved oxygen
Ozone can increase water soluble oxygen in two ways:
1: Reduction of oxygen-reactive compounds
2: Conversion of residual ozone to dissolved oxygen
The ozonation system usually increases the amount of dissolved oxygen by up to 2 units with any amount of inlet or outlet water discharge, provided that the appropriate dose of ozone is administered and injected. Interestingly, ozone solubility in water is ten times higher than oxygen in water.
Does ozone affect viruses?
Viruses are non-autonomous particles that propagate only inside the host cells. Each virus contains a nucleic acid molecule. Around this molecule is a protein coating called capsid.The capsid’s function is to protect the nucleic acid and also allow it to bind and penetrate the host cell.The mechanism of ozone destruction by the virus differs from that of other microorganisms. so that destroying the virus’s nucleic acid by penetrating ozone molecules through the protein coating. And by increasing the amount of ozone injected by destroying the capsid protein, it completely destroys the viral cell. Ozone has the ability to eliminate rabdo viruses.