water quality & fish hygiene
The chemistry of natural surface waters is complex, and depends on the equilibrium reached with the normal physical, chemical and biological characteristics of the surrounding environment. Thus, there can never be a normal surface water quality; every natural water will have a different composition.
Even rainwater varies in composition in different localities and regions. The precipitated water droplets will absorb acidic ions, volatile chemicals and fine particulate matter of natural and anthropogenic origin. A substantial proportion of the nitrogen input to soils comes with the winter rainfall. Recent data have shown that the level of atrazine in rain can be as high as 1 μg l-1 in areas where there is widespread use of this herbicide.
Rain that falls on soil overlying granite rock will tend to remain acidic and the run-off will be a soft water, low in calcium and bicarbonate. Water draining from areas of peat bogs will also be acidic, and can be extremely so with sudden rainfall after a prolonged dry period. Water with a low pH (<5.0) will dissolve naturally occurring metals from the soils and rocks, especially aluminium and in some areas copper, zinc and lead. Soft waters may be clear, or brown with varying amounts of dissolved humic substances.
Water falling on soil overlying chalk and limestone will become alkaline with a hardness depending on the amount of dissolved calcium and bicarbonate that it contains.
To some extent the surface water quality will depend on the type of vegetation covering the watershed, since the products of plant decay (as with the peat bogs mentioned above) will find their way into the .streams draining the area. Water draining from coniferous forests tends to be acidic
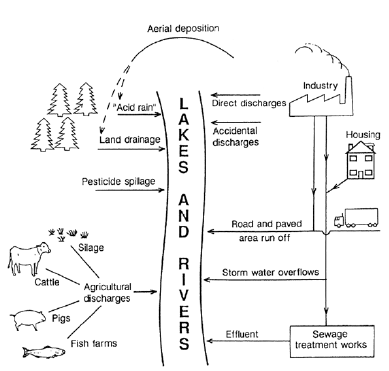
These are examples of natural causes of differences in water quality. Some indication has already been given of the added impact that can be caused by man’s activities. Metal mining, by increasing the surface area of exposed rock to rainfall, can cause elevated concentrations of metals in drainage water. Commercial forestry can cause an increase in suspended mineral solids in the water after areas have been cleared by cutting and logging. Intensive agriculture can contribute fertilizers and pesticides applied to arable crops, and strong organic liquids can be produced from silage manufacture and from the rearing of poultry and cattle. Weaker organic wastes, but in much greater volumes, can be discharged in the form of untreated or treated sewage.
These various man-made inputs are shown in Fig. 1. A broad distinction can be made between point-source inputs which originate from pipe-line discharges, and diffuse inputs which can occur over a wide area.
As well as these changes in water chemistry, the physical habitat of the watercourse can be altered by man’s activities. Rivers can be canalized for transport and flood prevention; dams are constructed for water storage and power generation; diversions are made to accommodate other land usage projects. These changes can affect the flow and depth of the water. Also, changes in the drainage characteristics of the watershed can .lead to a more rapid run-off, leading to a greater fluctuations in the river flow rates
Fig. 1: Sources of man-made inputs into the aquatic environment (from Lloyd, 1992)
Mention should be made here of the diurnal and seasonal fluctuations of temperature. These are less susceptible to man’s intervention, although changes in water depth and flow rates can affect the rates of diurnal warming and cooling. Obviously, heated discharges from power stations and industry can have a considerable effect on the aquatic biota.
Apart from affecting the suspended mineral solids content of the water, these physical changes per se have little effect on the chemical quality of the water. However, they can affect the natural biological community that can live in equilibrium with the particular chemical and physical characteristics of their environment, and changes in the composition of the aquatic biota can also affect the water quality.
The most obvious effects are those caused by increased plant growth. Clearly, rooted plants will provide shade and cover for a wide range of aquatic species. But all green plants, including algae, photosynthesize during their period of growth in the daylight hours, and respire at all times. During daylight, plants absorb carbon dioxide from the water and this is converted to carbohydrate; dissolved oxygen is produced and this is released into the water.
When plant growth is active, these photosynthetic processes are more pronounced than that of respiration, in which dissolved oxygen is absorbed and carbon dioxide is released. As a result, the pH of the water is raised during the day as the amount of carbonic acid is reduced; the dissolved oxygen concentrations are also raised during the day. At night, the level of carbon dioxide increases, leading to a lower pH, and the level of dissolved oxygen falls.