Dissolved oxygen in fish breeding
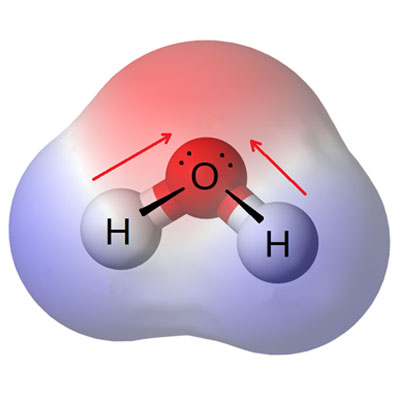
Oxygen enters the water through passive diffusion into the atmosphere and also enters the water through photosynthesis of phytoplankton and other aquatic plants. Water-soluble oxygen is essential for the breathing and life of aquatic plants and animals.Often times water-soluble oxygen is expressed in milligrams per liter. The amount of dissolved oxygen in water depends on three important factors: water temperature, sea level and salinity. For this reason, a small amount of oxygen in water can be dissolved, which is due to atmospheric pressure. The diagram below shows the inverse relationship between temperature and dissolved oxygen. If the temperature rises, the water dissolved oxygen decreases and increases at low temperature.The maximum dissolution capacity of oxygen is 100% oxygen saturated in water and the excess oxygen is released from the water into the air. It can also be increased up to 5 times if you use industrial oxygen maker packages. The optimum dissolved oxygen in cultivated water is near saturation (130%). The amount of optimal and tolerable dissolved oxygen varies during fish growth stages. Oxygen ranges between 7 and 10 mg / L during the incubation period and early stages of fish growth. Suitable for other growth stages of water-soluble oxygen in the range of 12-12 mg / l. It is important to know that fish consumption of oxygen increases strangely during eating and after eating. During this period, the amount of oxygen consumed increases significantly.
The role of dissolved oxygen in the corrosive property of water:
Water-soluble oxygen is one of the main causes of water’s corrosive properties. Iron and oxygen in contact with water form cathode and anode poles.
Iron acts as an anode, converted to ferrous hydroxide and then ferric hydroxide and then to iron oxide. Due to the numerous problems caused by the presence of oxygen in industrial waters (especially in boiling water), it is necessary to remove them from industrial use waters.
Oxygen Consumers in the breeding pool Including:
Tip
Fish need 3 times more oxygen than usual to complete their digestion.
Feeding should be based on the percentage of body weight of the fish to prevent food from remaining in the pool.
Feeding should be arranged in a timely manner so that the fish will not be deprived of oxygen during digestion, so it is important to avoid feeding in the morning and at sunset.
It is essential to provide dissolved oxygen when reducing oxygen, especially from sunset to before sunrise.
Photosynthesis
The intensity of photosynthesis in fish ponds is a function of the density of phytoplankton and the intensity of light at water depth.
Assuming that radiation intensity is constant, the amount of phytoplankton density will determine the amount of photosynthesis in water, while increasing the density of phytoplankton leads to a decrease in light penetration in water, which reduces the intensity of photosynthesis. And so the intensity of photosynthesis will be limited to the shallower areas.
The amount of dissolved oxygen in water is inversely proportional to 3 factors
If the water temperature is 15 ° C and the height of the free surface is 1200 m, the amount of dissolved oxygen in the water is 5.8 mg / l.
The amount of dissolved oxygen in addition to water temperature factors – water hardness and salinity – water height should also be proportional to the density of fish in the pools. It is essential that the oxygen in the outlet water of the eggs and larvae retention troughs should not be less than 7 mg / l.
Dissolved oxygen (DO) is essential for the breathing of aquaculture species.Oxygen is soluble in water from atmospheric fish ponds as well as photosynthetic operations. The oxygen in the air penetrates and dissolves in the water, but this process happens slowly.
The most important source of dissolved oxygen in photosynthetic fish ponds is highly dependent on the amount of light available to aquatic plants (phytoplankton); Therefore, dissolved oxygen production decreases on cloudy days and ceases at night and decreases, with increasing water depth based on the amount of turbidity.Therefore, to increase the dissolved oxygen, specialized equipment in the field of fisheries can be utilized, in addition to designing a system for transferring water to fish ponds in such a way that the water at the beginning of the installations and structures will be substantially dissolved in oxygen.
Natural waters usually contain some dissolved oxygen.
The life of aquatic plants depends on the minimum concentration of water-soluble oxygen.Fish require more dissolved oxygen than most other organisms and bacteria less than all aquatic life. The amount of dissolved oxygen in the water in which the fish are reared should not be less than 5 mg / L. If the amount of oxygen in the water is less than the minimum allowed for aquatic life, it is considered contaminated.
The need for salmon to oxygen depends on the following factors
- Fish size: The smaller the fish the more oxygen it needs.
- Fish activity: Increased oxygen demand during activity and mobility after eating.
- Temperature: Fish need more oxygen as a result of rising temperatures.
- Stress: Increases oxygen consumption.
Symptoms of oxygen deficiency are detectable by changes in fish behavior. Behaviors such as coming to the surface of the water in the inlet flow path if this condition persists for a long time can lead to the death of the fish.
Reduction of dissolved oxygen in water depends on:
Quiet, cloudy and warm weather
On cloudy days or days when the air is misty, sunlight does not reach the surface of the water, and photosynthesis and oxygen production by phytoplankton is not achieved, resulting in reduced water oxygen.
Pools that have a high planktonic boom at the water’s surface do not penetrate the sunlight, resulting in oxygen being produced only at the water’s surface and not penetrating the lower layers. Encounter oxygen deficiency.
As the temperature increases, the amount of oxygen in the water decreases as the activity of the organisms increases with increasing temperature. Fish are cool creatures so their body temperature and activity are affected by the water temperature. Stopping the flow of wind also causes the oxygen in the air to not enter the water through the emission.
Sudden death of phytoplankton or algae
When phytoplankton and algae die in water, there is also a lack of oxygen in the water because not only are the oxygen sources in the pool depleted, but also their dead algae and phytoplankton consume oxygen.
During dark hours, high densities of phytoplankton can consume oxygen in the water, which is used to breathe fish.
Pool water layering
During the summer, the upper layer of water (1 m deep) is heated very quickly at the depth of the pool (2–3 m) and its density is reduced and lighter than that of the pool floor.
Because the water on the top layer is warmer and lighter, it cannot mix with deep-water cold water, and the cold water remains immobilized near the bottom and its oxygen is reduced and toxic compounds are produced by bacteria and decaying organic matter.
If the weather is cold or it rains, the top layer will coincide with the bottom layer and the water will mix and there is no problem with the lack of oxygen in the bottom.
Organic matter decomposition of bottom of the pool
As the biomass (total fish weight) in the ponds rises (summer), the organic matter and fecal load of fish (ammonia, nitrate, stool, and digestible food) increase.
Organic and fecal matter of the fish decomposes and increases the oxygen demand of the fish. Organic matter and fish feces can also stimulate algae growth in the pool.
Fish requirements for oxygen
The absorption and excretion of oxygen by hemoglobin is due to the surface tension of oxygen. In gills, the surface tension of oxygen in the water is higher than blood, so oxygen flows through the water to lungs and is absorbed on the hemoglobin. This happens in the opposite way to the receive and moves in the oxygen tissue from the blood with high surface tension of dissolved oxygen to the tissue.
The interactions between dissolved oxygen and the percentage of hemoglobin saturation of oxygen are called the dissociation curve of oxyhemoglobin.
The shape of this curve is either sigmoidal or S-shaped and hyperbolic.
In hydrothermal fish, this curve is usually hyperbolic, and in tropical fish is sigmoid.
This means that these fish can carry much more oxygen when the dissolved oxygen has minimal surface tension.
The amount of oxygen consumed in the fish depends on their age, species size, activity and respiratory status.
It is generally estimated that fish consumption for freshwater fish species at resting temperatures is about 17 to 20 ° C at about 65 to 210 mg / l.
According to colleagues’ research in 1976, If soluble oxygen concentrations reach less than 25% saturation, fish growth will decrease.
Because they are 100% saturated, the carrier proteins are 100% saturated.
In addition, over saturation can lead to gas bubble disease.
Fixing the Problem of Oxygen Deficiency in Pool Water
To increase the amount of dissolved oxygen can be:
- A variety of mechanical aeration equipment can be used in any pond.
- First, water purification and transport equipment should be designed in such a way that the water will be sprayed on arrival at the facility.
- The ponds can be designed in such a way that their length is in the direction of possible winds.
Ways to control oxygen concentration in the pool
A) Aeration
B) Change the fertility status of the water by controlling the plankton, especially when the seasons change.
C) Increase inlet water flow
D) Proper design of breeding fish ponds.
Factors Indicating Lack of Oxygen
- Fish skin pale
- Gill bleeding
- Small bleeding in some parts of the fish’s body
Measurement of dissolved oxygen
Water-soluble oxygen should be monitored twice a day, early in the morning and early in the night. Low oxygen levels early in the night are very worrying.
Tips
Larger fishes have higher oxygen consumption as a result of fish coming to the surface being louder. The use of an oxygenator is essential when reducing oxygen, especially from early night to early morning.
Predict the amount of dissolved oxygen in the pool
It is imperative for management to predict the amount of dissolved oxygen in the pool.
Dissolved oxygen concentration at dusk
Inlet and outlet oxygen through diffusion
Consumption of oxygen by low-level organisms
Fish consumption of oxygen
Oxygen consumption by the phyto-plankton community
Considering this equation, it surprisingly predicts the amount of oxygen in the pool.
Benthic respiration has the least role in depletion of water-soluble oxygen.
Influence of suspended particles on the amount of dissolved oxygen in the pool water:
Sometimes with insufficient oxygen in the water, there is insufficient oxygen for the metabolic activity of the tissues. This is called tissue hypoxia.
Its causes can include parasite infestation, infectious gill disease, excessive mucus secretion, pH beyond fish tolerance. And also some pesticides that contain some metals like copper and zinc.
Physical factors affecting the amount of water dissolved in oxygen
- Temperature: The amount of dissolved oxygen in the water varies with the temperature of the water. As the water temperature rises, the amount of dissolved oxygen in the water will decrease.
- Salinity: Increasing the salinity of the water reduces the degree of oxygen saturation, which results in less oxygen retention in the water. Salmon can be bred in a wide range from freshwater to seawater, but their survival and growth are reduced to about 20 g / l salinity (PPt).
- Atmospheric pressure or above sea level: As air pressure increases, the degree of saturation of dissolved gases, including oxygen, will increase. The higher the sea level, the lower the amount of dissolved oxygen in the water.
How much air is needed
Depending on the density and amount of production, for example, under normal conditions we need three to four aeration units for every 6 to 9 tons of fish or 3 to 6 tons of shrimp.
What kind of aeration do we use
For indoor pools of about 2 meters or more, the best aeration is Turing Turbine Devices, which are placed in the proper depth and flow by sucking them out and injecting them into the water.
Aeration systems in fish farming
One of the benefits of aeration besides producing oxygen is that it also mixes water and balances the thermal and nutrient layers and also removes the aeration action of toxic wastes that are soluble in water.
What is aeration and what is the benefit?
Aeration is a method by which water-soluble oxygen is equilibrated with air (saturation). This is done by mechanical devices.
Any device that can mix with the air is a type of aeration, but because of the efficiency of the work and the energy consumption and the cost of running it is very important that we use a special aeration system for the aeration.
One is injection of pure air or oxygen into the water (such as air pump, blower, etc.), and the second is to stir water or spout to balance oxygen.
So aeration is also a kind of mixing of water with the production of oxygen and uniformity of its thermal and nutritional layers. Aeration also helps to remove toxic wastes that are soluble in water as a gas.
In general, the selected aeration should have the following benefits:
1- Style
2- No need for grease
3- Permanently working
4- Cheap
5- No sophistication in its technology
6- Oxygen transfer efficiency compared to energy consumption
Splash aeration device
Advantages of Splash:
Easy maintenance, installation
Low power consumption compared to similar aeration devices
Suitable for any type of pool because of the adjustable height of the impeller
Floating with PE insulation and resistant to corrosion, shock and sunlight
Low price
Disposal of harmful gases
Ability to connect three or single phase power
Airjet aeration machine
Another powerful aeration device for mixing weather with one another is the Airjet, The mechanism of this device is that an electromotor is connected to a blade inside a hollow tube, The system is mounted on a floating subject and at the time of powering the electric motor the impeller rotates and suck air into the water. One of the important advantages of Airjet is the ability to adjust the aeration paths at different depths for more oxygen transfer, and because of its buoyancy, water fluctuations do not affect the performance of the device.
Benefits of Airjet Air Conditioning
Easy to move
Mixing heat and food layers
Prevent thermal stratification
Increase water soluble oxygen
Suitable for use in 1.5m deep pools, especially in tropical and hot water pools
Blower aeration device
Deep or central aeration is carried out by one or more blowers or side channels.Due to its high capacity at low pressures produced by the blowers, this blower has been welcomed by many aquaculture producers. These blowers are capable of being used as boosters and are driven by fittings and pipes to the breeding fish ponds and continue to the pool floor, which eventually forms into multiple branches on each branch of the diffusers. It is installed to convey air into tiny bubbles of water. Using this aeration system increases the job reliability and work efficiency. It is very important to use proper diffusers for aeration to prevent them from being jammed and damaged.
The most reliable and widely used aeration system is the use of blowers and siding channels with the help of diffusers.
Oxygen transfer in two ways: photosynthesis and air diffusion
Oxygen required for pools is provided by photosynthesis and diffusion by air. Photosynthesis means the plant uses food and oxygen. Plants exposed to sunlight add oxygen to the water from photosynthesis; One thing to note is that plants do not produce oxygen at night, and with the consumption of oxygen by fish and bacteria, the concentration of dissolved oxygen in the water decreases, causing stress to the aquatic species and even causing them to die. There is a large difference in the amount of oxygen present in the pools at different times, although the oxygen concentration reaches its lowest level in the morning and by the end of the afternoon reaches its highest level. Maximum oxygen retention depends on atmospheric pressure, salinity, and water temperature. As the altitude rises, the oxygen level of the water decreases.
Water salinity is not important to most freshwater fish producers and is the most important and influential factor in water temperature. The higher the water temperature, the lower the oxygen storage capacity.
PSA oxygen production technology
One method of production is at the site of consumption, Production in PSAs is based on the passage of compressed air through the molecular air contained in the generator tanks, in which the air molecules are separated under pressure, and this is done according to the type of oxygen generator. Note that air separation is a physical process where no chemical interactions occur and as soon as the pressure is removed from the molecular sieve material, the molecular regeneration is regenerated again. Therefore, PSAs are a good replacement for commercially available cylinders if used correctly. Generator design is based on international standards with the aim of optimizing energy consumption, environmental compliance and working in different climates. The use of generators, while ensuring convenience and continuous supply of oxygen, returns the capital used to purchase the system in the short term.
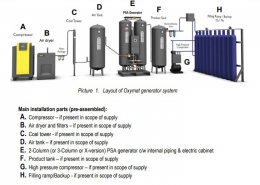
The components used in the oxygenation system include:
1. Compressor
Oxygenation systems require a compressor to supply compressed air to deliver compressed air.
- The compressed air produced by the compressor has heat and some moisture, which, if logged in, will cause the zeolite to die off early. For this purpose, a dryer or air dryer is provided in the system.
- Compressors use the surrounding air to produce compressed air. In the ambient air, there are lots of hydrocarbon particles, bacteria and water vapor. These impurities can be eliminated by passing pneumatic equipment.
2. Dryer
3. Filtration
Filterings include:
- GRADE AO : Ability to absorb water and oil particles up to 1 micron.
- GRADE AA*2: Ability to absorb water and oil particles up to 0.1 micron.
- GRADE ACS : Ability to absorb water and oil particles up to 0.003 micron.
As well as an antibacterial filter and an activated carbon filter installed after the oxygen tank.
- The compressed air tank and system oxygen store the required system air as well as the oxygen needed for peak times of consumption and cause the least changes in system pressure.It has a safety valve and pressure indicator. These tanks are also painted in an industrial way with an inner coating of epoxy paint to prevent decay.
4- Oxygen generator
Oxygen generators
Monitoring system for easy operation of the machine operator Display of purity, ambient temperature and tank pressure at any moment
Ability to display oxygen and nitrogen production process on 7-inch full color monitor and display on PC and mobile
Automatic instantaneous and continuous measurement of pressure, temperature and purity of oxygen and nitrogen in digital, analog and linear state with storage capacity
Use a transmitter to control the pressure of the tanks so that the pressure and purity can be calculated at any moment by the PLC and displayed on the monitor screen.
Using Italy’s Pneumatic VIP Valves instead of Solenoid Valves to extend service life and reduce explosion risk
Fully automatic switchgear for transferring consumption line from oxygen generator to back-up capsule during emergencies
Molecular used in generators from the best and most sought after brands in the world
ZIRCONIUM Lifetime Oxygen Sensor with Automatic Calibration, Permanent Working
Valves from stainless steel SS316L material
All substrates have been sandblasted under pressure which prolongs the life of the tanks.
5. Air and oxygen tanks
The Benefits of Pure Oxygen in the Aquaculture Industry:
Increase the concentration of dissolved oxygen up to 4 times its saturation of about 40 mg / L, especially in hot waters in summer.
Increase aquatic production per unit volume
Reduce risk and prevent fish deaths in summer as well as in warm waters.
Increase aquatic production by up to fivefold in areas with limited water resources or rotary systems are used for aquaculture.
Better aquatic nutrition and Significant decrease in aquatic growth period
Decrease in aquatic excrement material
Significant reduction in water intake in rotary systems
Reduce common infections
Reduce consumption of tonic material to increase fish weight.
Suitable oxygenation in Race way systems during river water pollution
Prevents the saturation of nitrogen gas in water and prevents gas bubble disease
No effect of geographical fluctuations (height, pressure and temperature) on the capacity of the device and storage system.
Eliminate all costs related to providing, transporting, storing and recharging cylinders and oxygen tanks.
Equipped with a sensitive controller for detecting the compressed air entering the system as well as having an overview of the purity and pressure of the output gas.
LIQUID OXYGEN
There are two ways to convert oxygen from gas to liquid:
- Increased oxygen pressure: Oxygen can be stored and transported at pressures of 831 bar or 200 psi in 1 to 60 liters capsules.
- Reduce oxygen temperature: At temperatures below -147 ° C, it can be transported as cryogenic liquid in special tankers.
Production of pure oxygen in the place of consumption:
The most common methods of producing pure oxygen on site are:
1- Separation of oxygen atoms from water molecules by electrolysis process
2- Oxygen production through the separation of constituent gases
Separation of oxygen atoms from water molecules
This separation is accomplished by electrolysis of water molecules.
The main purpose of this method was to produce hydrogen with respect to the volume ratio of oxygen and hydrogen atoms in the water. After separation of this element, the resulting oxygen gas, having a purity of 98-99%, was stored in a cylindrical tank with a capacity of 3–7 m3 with a pressure of 2550 psi. Are quoted. Due to its high cost and cost, it is used only as a supporter of other oxygen production methods.
Production of oxygen through the separation of the constituent gases of air
Given the high percentage of air composition (about 78%) of nitrogen, oxygen can be produced by separating nitrogen gas from the air. The following methods are used:
A) Distillation method (Liquid Oxygen Generation or LIQUID OXYGEN):
The mechanism of pure oxygen production in this system is the use of cryogenic refrigeration cycle, which separates the gases based on their boiling point difference. Liquid oxygen is produced in large and medium-sized units of oxygen and nitrogen by the distillation of liquid air.
This procedure involves the following steps:
- Filtering and compressing the air
- Separation of pollutants, air vapor and carbon oxides from the air
- Cooling of air by heat exchanger and cooling
- Fluidize the air to a temperature of about-196 degrees Celsius
- Heating gaseous products and their gradual separation from liquid air
The purity degree of oxygen produced by this method is 98-99%.Liquid oxygen is transported by special tankers and discharged into storage tanks for this purpose. Storage tank pressure can be varied from 150 to 200 psi and range from 100 liters to over 40 cubic meters; Liquid oxygen must be converted to gas before consuming by passing through the spiral tubes. The system consists of components such as storage tank, steamer, filter and pressure regulator. It is less used because of the hassle of shipping and its high costs.
The advantages of this method are the need for no electricity, two storage tanks and a low risk of explosion.
A) Pure oxygen production technology by VSA method (Vacuum swing adsorption):
The VSA package is made up of three separate parts that do not have a compressor and dryer and blowers are used instead of compressors. At all stages of oxygen production, air passes through the zeolite bed, the zeolite grains being about two millimeters in diameter capable of absorbing nitrogen molecules as the oxygen molecules pass through their lids and are collected in a storage tank. Nitrogen is also combined with carbon dioxide and discharged from the system. One of the advantages of this method is that the zeolite grains are not contaminated with oil and water droplets and so on because of the low pressure and the lack of compressor. The purity of the oxygen gas produced can be up to 90% and the flow rate between 150 and 500 liters per minute.
B) Separation of oxygen from air by zeolite granules:
The most common methods of producing pure oxygen at the site of consumption include producing oxygen by PSA and VSA methods; In both methods of molecular sieves zeolite for purification and it is used to separate oxygen gas from the air. The principles and mechanism of work in both methods are similar except that the equipment used in the VSA method is simpler and the number of equipment used is far less than the PSA method; For this reason, in the VSA method, the system reliability is higher and its maintenance costs are much lower than the PSA. In the PSA method, it is also possible to separate oxygen from other air gases at high pressure (about 8 bar), which requires such a high pressure to require a compressor with good power and dryer, while at VSA this is performed at low pressure (about 1.5 bar). It can also be supplied with a blower.
Factors and Influences in Estimating the Type, the amount, and where to install aeration devices:
1- The relative weight of fish in the pool
2- The shape and size of the pool
3- The amount of Inlet fresh water
4- Chemical and physical specifications of water
5- Sea level in fish farming
6- Amount of fish density and general status of the pool
Tip:
Providing oxygen to fish is sometimes more important than providing food.
Because :
The presence of oxygen in the water causes digestion and absorption of food, so oxygen can improve the feed conversion ratio.
With enough oxygen in the water, you can store more water per unit area and feed more so you can produce more per unit area.
Sufficient oxygen in the water prevents water from being poisoned by aquatic waste or even substances entering the water from outside.
Oxygen in the water brings more health and vitality to the fish.
Three major reasons for using oxygen in aquaculture:
Healthy product
Fast growth
High density
The importance of Oxygen supply for aquaculture
1- Reduce feed conversion ratio or increase digestion and absorption of food
2-Increasing water efficiency, thereby more production
3- Prevent water poisoning by substances that enter the water from outside as well as aquatic waste
4- Increase aquatic growth, health and vitality
Tip:
Providing oxygen to fish is sometimes more important than providing food.
Because:
The presence of oxygen in the water causes digestion and absorption of food, so oxygen can improve the feed conversion ratio.
With enough oxygen in the water, we can store more aquatic per unit area and feed more so you can produce more per unit area.
Sufficient oxygen in the water reduces the toxicity of water by aquatic waste or even substances that enter the water from outside.
Oxygen in the water brings more health and vitality to the fish.