CAUSES AND EFFECTS OF POLLUTION ON FISH
3.1 Harmful variations in natural water quality characteristics
3.1.1 Water temperature
3.1.2 Water pH
3.1.3 Dissolved oxygen
3.1.4 Supersaturation with dissolved gas
3.1.5 Ammonia
3.1.6 Nitrites and nitrates
3.1.7 Hydrogen sulphide (H2S)
3.1.8 Carbon dioxide
3.1.9 Summary
Harmful variations in natural water quality characteristics
The following physico-chemical changes in the aquatic environment are those most frequently recorded as the primary causes of harm to fish in fish culture installations.
considers the important factors involved on an individual basis and in greater detail.
Water temperature
Fish are poikilothermic animals, that is, their body temperature is the same as, or 0.5 to 1°C above or below, the temperature of the water in which they live. The metabolic rate of fish is closely correlated to the water temperature: the higher the water temperature (i.e. the closer to the optimum values within the normal range), the greater the metabolism. This generalisation applies particularly to warm-water fish. Cold-water fish, e.g. salmonids, whitefish, or burbot, have a different type of metabolism: their metabolic rate can continue at comparatively low temperatures, whereas at high water temperatures, usually above 20°C, they become less active and consume less food. Water temperature also has a great influence on the initiation and course of a number of fish diseases. The immune system of the majority of fish species has an optimum performance at water temperatures of about 15°C.
In their natural environment, fish can easily tolerate the seasonal changes in temperature, e.g. a decrease to 0°C in winter and increase to 20–30°C (depending on species) in summer under Central European conditions. However, these changes should not be abrupt; temperature shock occurs if the fish are put into a new environment where the temperature is 12°C colder or warmer (8°C in the case of salmonids) than the original water. Under these conditions fish may die, showing symptoms of paralysis of the respiratory and cardiac muscles. With young fry, problems may arise even where the difference in temperature is as low as 1.5–3°C. If fish are fed and then abruptly transferred to water colder by 8°C or more, their digestive processes will slow down or stop. The food will remain undigested or half-digested in the digestive tract and the gases produced can cause the fish to become bloated, lose balance, and finally die. If carp are given a high-nitrogen feed (e.g. natural food or high-protein pellets), abrupt transfer to much colder water will considerably increase the level of ammonia nitrogen in the blood serum because the decrease in metabolic rate reduces the diffusion of ammonia from the gills. This can lead to ammonia autointoxication and death.
Considerable progress has been made recently in warm water fish culture. Techniques for water temperature control enables optimal condition to be maintained, so that the fish can fully utilize their growth potential to achieve maximum weight gains
Supersaturation with dissolved gas
Supersaturation with dissolved gas occurs when the pressure of the dissolved gas exceeds the atmospheric pressure. It occurs when water is equilibrated with air under pressure, e.g. at the bottom of a lake or reservoir, in ground water, or if air is drawn into a centrifugal water pump. It can also occur if cold air-equilibrated water is warmed up without re-equilibration to the higher temperature. A bottle containing such water will show either minute bubbles forming as a cloudy suspension which will clear from the bottom upwards, or larger bubbles forming on the glass wall. This is analogous to that seen in an opened bottle of carbonated drinking water.
If fish are exposed (at a lower atmospheric pressure) to such water, their blood equilibrates with the excess pressure in the water. Bubbles form in the blood and these can block the capillaries; in sub-acute cases the dorsal and caudal fin can be affected, and bubbles may be visible between the fin rays. The epidermal tissue distal to the occlusions then becomes necrotic and cases are known where the dorsal fins of trout have become completely eroded. In severe cases, death occurs rapidly as a result of blockage of the major arteries, and large bubbles are clearly seen between the rays of all the fins. A similar effect of gas bubbles forming in the blood can be experienced by deep-sea divers when they return to the surface.
The remedy is either to remove the fish to normally equilibrated water or to provide vigorous aeration to strip out the excess gas.
Water pH
The optimal pH range for fish is from 6.5 to 8.5. Alkaline pH values above 9.2 and acidity below 4.8 can damage and kill salmonids (e.g. brown and rainbow trout); and pH values above 10.8 and below 5.0 may be rapidly fatal to cyprinids (especially carp and tench). Thus salmonids, in comparison with cyprinids, are more vulnerable to high pH and more resistant to low pH. The American char is especially resistant to acid waters and can tolerate pH levels as low as 4.5–5.0.
Low water pH most frequently occurs during the spring, especially when acidified snow melts, and in water draining peat bogs. High alkaline pH can occur in eutrophic reservoirs (ponds) where the green plants (the blue-green algae, green algae and higher aquatic plants) take up considerable amounts of CO2 during the day for intensive photosynthetic activity. This affects the buffering capacity of the water and the pH can rise to 9.0–10.0 or even higher if bicarbonate is adsorbed from waters of medium alkalinity. Water pH can also be changed when mineral acids and hydroxides, or other acidic or alkaline substances, are discharged or leach into water courses, ponds or lakes.
As a defence against the effect of a low or high water pH, fish can produce an increased amount of mucus on the skin and on the inner side of the gill covers. Extremely high or low pH values cause damage to fish tissues, especially the gills, and haemorrhages may occur in the gills and on the lower part of the body. Excess amounts of mucus, often containing blood, can be seen in post mortem examination of the skin and gills. The mucus is dull-coloured and watery.
Water pH also has a significant influence on the toxic action of a number of other substances (e.g. ammonia, hydrogen sulphide, cyanides, and heavy metals) on fish.
Ammonia
Factors associated with ammonia toxicity
Ammonia pollution of water courses, ponds and lakes may be of organic origin (domestic sewage, agricultural wastes, or the reduction of nitrates and nitrites by bacteria in anoxic waters) or of inorganic origin (industrial effluents from gas works, coking plants and power generator stations). In water or in biological fluids, ammonia is present in a molecular (nondissociated) form (NH3) and in the form of ammonia ion (dissociated) (NH4+). The ratio between these two forms depends on the pH and temperature of the water (Table 1). The cell walls of organisms are comparatively impermeable to the ammonia ion (NH4+), but molecular ammonia (NH3) can readily diffuse across the tissue barriers where a concentration gradient exists, and is therefore the potentially toxic form to fish. Also, under normal conditions there is an acid-base balance at the water-tissue interface. If this balance is altered, the side on which the pH is lower will attract additional molecular ammonia. This explains how molecular ammonia passes from water through the epithelium of the gills to the blood and also how it passes from the blood to the tissues. Ammonia has a particular toxic effect on the brain; this is why nervous symptoms are so pronounced in cases of ammonia toxicity to fish.
Water quality monitoring of water courses, lakes and fish culture facilities includes the measurement of total ammonia concentrations. To assess the potential toxicity of these concentrations it is important to know the amount of nondissociated ammonia (NH3) present. This is calculated from the measured values for total ammonia (NH4++NH3), temperature (T, °C) and water pH, using the formula :
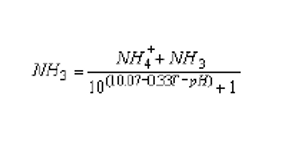
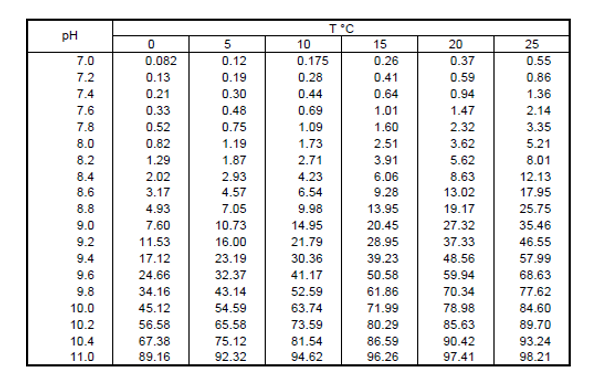
Alternatively, the values can be interpolated from Table 1 compiled from calculations on the basis of this formula.
Table 1: The NH3 content (as % of total ammonia) of water at different pH values and temperature
Besides water temperature and pH, other factors that influence ammonia toxicity include the concentration of dissolved oxygen in water; the lower the oxygen concentration in water, the greater the toxicity of ammonia (Fig. 4).
To a lesser extent, the toxicity of ammonia is affected by the amount of free CO2 in the water. This is because the diffusion of respiratory CO2 at the gill surface reduces the pH of the water, thus reducing the proportion of nondissociated ammonia there. The extent of the reduction in pH depends on the amount of CO2 already present in the water.
In general, Table 1 shows that the toxicity of ammonia will be much greater in warm alkaline waters than in cold acid waters.
Non-dissociated ammonia is highly toxic to fish. The LC50 values, determined in acute toxicity tests, are in the range of 1.0 to 1.5 mg NH3 per litre for cyprinid fish and 0.5 to 0.8 mg NH3 per litre for salmonids. The maximum admissible ammonia (NH3) concentration is 0.05 mg per litre for cyprinids and 0.0125 mg per litre for salmonids.
It should be emphasized here that these standards apply to ammonia as a toxic substance. Other standards for total ammonia are applied to control eutrophication of waters and prevent excessive algal and plant growth that can cause physical problems and affect the oxygen balance.
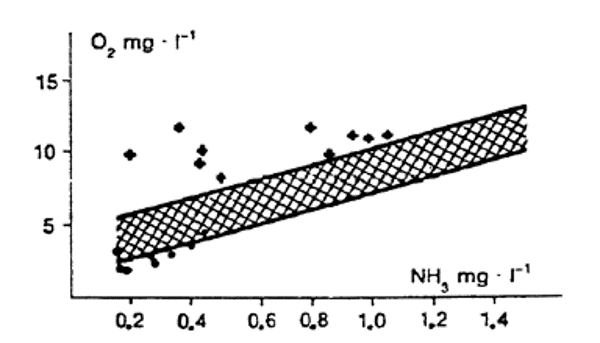
Fig. 4: With low levels of oxygen in the water, lower concentrations non-dissociated ammonia can kill fish:
- fatal cases; + waters where no cases of injury to fish occurred in March to April; hatched area = lethal boundary of non-dissociated ammonia (Vámos and Szöllözy, 1974)
The first signs of ammonia toxicity include a slight restlessness, and increased respiration; the fish congregate close to the water surface. In later stages, cyprinids gasp for air, their restlessness increases with rapid movements and respiration becomes irregular; then follows a stage of intense activity. Finally, the fish react violently to outside stimuli; they lose their balance, leap out of the water, and their muscles twitch in spasms. Affected fish lie on their side and spasmodically open wide their mouths and gill opercula. Then follows a short period of apparent recovery. The fish return to normal swimming and appear slightly restless. This stage is then replaced by another period of high activity; the body surface becomes pale and the fish die.
The skin of ammonia poisoned fish is light in colour, and covered with a thick or excessive layer of mucus. In some cases small haemorrhages occur, mainly at the base of the pectoral fins and in the anterior part of the ocular cavity. The gills are heavily congested and contain a considerable amount of mucus; fish exposed to high ammonia concentrations may have slight to severe bleeding of the gills. Intense mucus production can be observed on the inner side of the gill opercula, mainly at the posterior end. The organs inside the body cavity are congested and parenchymatous, and show dystrophic changes.
In recent years, considerable losses among farmed carp have been caused by the so-called toxic necrosis of the gills. The factors responsible for the occurence of this disease include ammonia poisoning in which the ammonia level in the blood is considerably increased. As stated earlier, ammonia is the final product of nitrogen metabolism in carp (as it is in other species) and most of it is excreted via the gills into the water. If the diffusion rate is reduced for some reason or another (high water pH, oxygen deficit, damaged gills etc.), the ammonia level in the blood will steadily rise, causing a condition known as autointoxication, which may lead to toxic gill necrosis in carp.
A very interesting case of autointoxication among carp yearlings (C1) where extremely high ammonia N levels were found in the blood serum occurred after their
transfer from a pond to well water in large aquarium tanks. Some of the fish caught and transferred during the morning exhibited typical symptoms of ammonia poisoning the following morning. These symptoms included considerable restlessness, increased respiration, leaping out of the water, uncoordinated activity, and tonic-clonic spasms of the muscles. The skin of the affected fish was light in colour; the gills were heavily congested, dark red and showed oedematous swellings (particularly severe on the edges of the gill filaments). It is known that ammonia toxicity is accompanied by an increase in the permeability of the fish epithelium to water, as measured by an increase in the flow of urine. If the kidneys cannot cope with the increased water influx, oedema is likely to occur. An increased water influx may also occur if the skin or the mucus coating of the fish is damaged by handling and during transport. The histopathological changes in the gills corresponded with what had been described for toxic necrosis of carp gills. The digestive tract of those fish with severe poisoning symptoms was filled with undigested food. On the other hand, fish that had cleared their gut (faeces found on the bottom of the tank, the gut almost empty), were free from symptoms of toxic damage. The average blood serum level of ammonia N in the fish with symptoms of poisoning was 3054 (2400–3600) μg per 100 ml of serum, whereas in the fish free of such symptoms the ammonia level was 825 (750–900) μg per 100 ml of serum. In the affected fish the autointoxication, associated with the considerable increase in the blood serum ammonia N level, was probably due to the persistence and absorption of the gut contents (natural food and high-protein feed pellets) of the carp exposed to environmental stress (confinement and reduced oxygen level during transport, and water temperature reduced by about 5°C).
On the basis of this case of ammonia poisoning of carp, some other unexplained incidents of rapid death among fish may be ascribed to a similar cause. Such events may occur mainly in carp farms where there is an intensive feeding with a high-nitrogen diet, if the fish are also exposed to other stresses caused by e.g. an abrupt oxygen deficit, or sudden changes in water temperature.
Nitrites and nitrates
Nitrites as a rule are found together with nitrates and ammonia nitrogen in surface waters but their concentrations are usually low because of their instability. They are readily oxidized to nitrate or reduced to ammonia, both chemically and biochemically by bacteria. Nitrates are the final product of the aerobic decomposition of organic nitrogen compounds. They are present in low concentrations in all surface waters. There is almost no nitrate retention in soil, so it is readily leached to watercourses, ponds and lakes. The main sources of nitrate pollution of surface waters is the use of nitrogenous fertilizers and manures on arable land leading to diffuse inputs, and the discharge of sewage effluents from treatment works.
Nitrite can be associated with ammonia concentrations in the water. In normal aerobic conditions, ammonia is oxidized to nitrite and then to nitrate by two separate bacterial actions. If the second stage of oxidation is inhibited by bactericidal chemicals in the water, nitrite concentrations will increase. This may be important in small ponds or aquaria where water is recirculated through a purification filter; the ammonia-oxidizing bacteria need to become established for the filter to function, and they may be affected by the use of antibiotics to control fish diseases.
The toxic action of nitrite on fish is incompletely known; it depends on a number of internal and external factors (such as fish species and age, and general water quality). The importance and role of these factors have been frequently studied and reviewed. Different authors often come to contradictory conclusions, and usually fail to offer a definitive explanation of either the mechanism of nitrite toxic action on fish or the modifying effects of different environmental factors.
It is now clear that nitrite ions are taken up into the fish by the chloride cells of the gills. In the blood, nitrites become bound to haemoglobin, giving rise to methaemoglobin: this then reduces the oxygen transporting capacity of the blood. The increase in the amount of methaemoglobin can be seen as a brown colour of the blood and gills. If the amount of methaemoglobin in the blood does not exceed 50% of the total haemoglobin, the fish usually survive. If the fish have more methaemoglobin in their blood (70–80%) they become torpid, and with a further increase in the methaemoglobin level they lose their orientation and are unable to react to stimuli. Nevertheless, the fish may still be able to survive because the erythrocytes in their blood contain the enzyme reductase which can convert methaemoglobin to haemoglobin. This process can return the haemoglobin to its normal level within 24–48 hours, if the fish are put into nitrite-free water.
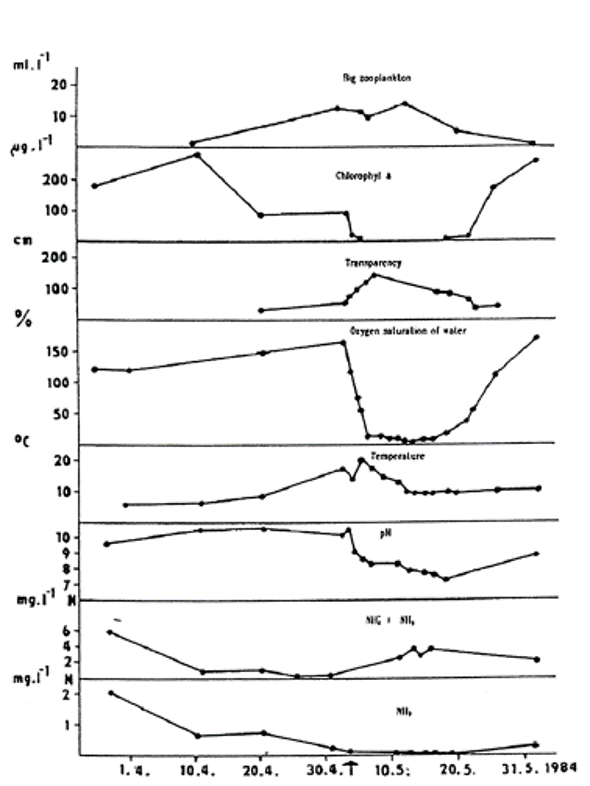
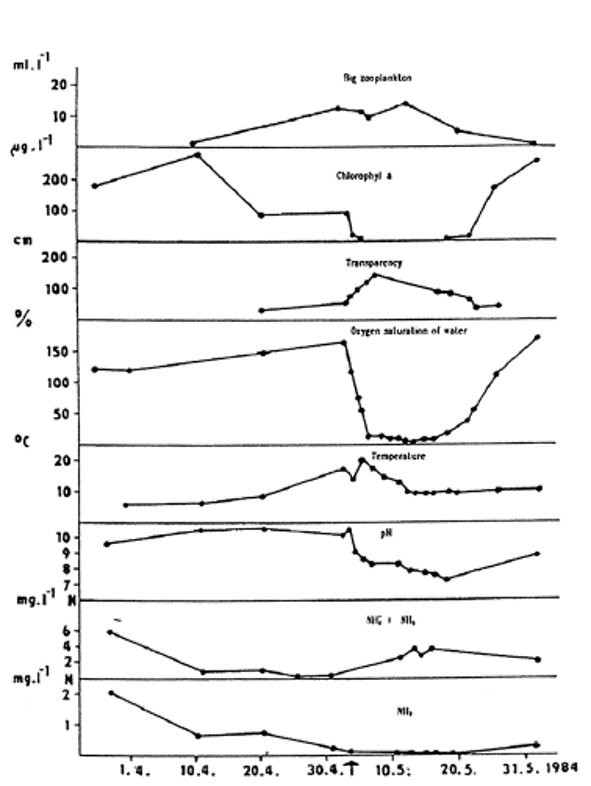
Fig. 5: Hydrobiological and hydrochemical conditions in the Dremliny Pond before and during the course of toxic gill necrosis in carp. Toxic necrosis was diagnosed on 2 May 1984.
Several authors have shown that nitrite toxicity to fish can be affected by certain water quality characteristics (e.g. Lewis and Morris, 1986). In this investigation, the 96h LC50 for rainbow trout ranged from 0.24 to 12.20 mg per litre, depending on the chloride content of the dilution water (in this case the chloride content ranged from 0.35 to 40.9 mg per litre). The effect of chloride on nitrite toxicity is so marked that the results of tests made without recording the chloride concentrations in the water cannot be compared with those of other tests.
It is now known that the chloride cells in the fish gills cannot distinguish between nitrite and chloride ions; both are transported across the gill epithelium. The rate of nitrite uptake depends therefore on the nitrite-chloride ratio in the water.
Nitrite toxicity can be also influenced by bicarbonate, potassium, sodium, calcium and other ions, but their effect is not so great as that of chloride. Among these, potassium is the more significant, and that of sodium and calcium is less. These
monovalent ions are also involved in the ionic fluxes across the gill epithelium and so directly or indirectly influence the uptake of nitrite. The pH value has also been considered as important for nitrite toxicity; pH and temperature control dissociation between NO2 and nondissociated HNO2 and it was believed that the uptake of nitrites into fish blood plasma depended on the diffusion of nondissociated HNO2 across the gill epithelium. However, the results of later experiments refuted these theories and showed that within the acidity-alkalinity range encountered in natural waters the pH is of little importance in nitrite toxicity.
Another factor that influences nitrite toxicity is the dissolved oxygen concentration and water temperature. This is associated with the fact that fish need a fully oxygenated water when the oxygen-carrying capacity of the blood is reduced by the formation of methaemoglobin, and the oxygen requirement of fish increases with temperature.
Long exposure to sublethal concentrations of nitrites does not cause much damage to the fish. Concentrations corresponding to 20–40% of the minimum levels having a lethal action on the fish may slightly depress their growth but no serious damage has ever been recorded.
For estimating the safe nitrite concentration for particular locations, it is necessary to measure the ratio of chloride to nitrite. These ratios (expressed as mg l-1 Cl: mg l-1 N-NO2–) are recommended to be no less than 17 for rainbow trout and 8 for fish of low economic importance.
The toxicity of nitrates to fish is very low, and mortalities have only been recorded when concentrations have exceeded 1000 mg per litre; 80 mg per litre is considered to be the maximum admissible nitrate concentration for carp and 20 mg per litre for rainbow trout. In surface waters and in fish farms where the water contains ample oxygen with no danger of denitrification (i.e. conversion of NO3– to NO2– and then to elementary nitrogen or N2O and NO), it is not so necessary to monitor the concentration of nitrates. However, as with ammonia, water quality standards need to be set for nitrate to prevent eutrophication, and the excessive growth of algae and plants, which can have a secondary effect on fish.
Hydrogen sulphide (H2S)
Hydrogen sulphide occurs in organically polluted waters from the decomposition of proteins. It is also present in industrial effluents including those from metallurgical and chemical works, paper pulp plants, and tanneries. It has a high to very high toxicity to fish; the lethal concentrations for different fish species range from 0.4 mg H2S per litre (salmonids) to 4 mg per litre (crucian carp, tench and eel). The toxicity of H2S decreases with increasing water pH, because of a reduction in the ratio of the nondissociated toxic H2S to the less toxic HS ions). The concentration of nondissociated H2S can be calculated from the measured total hydrogen sulphide (HS + H2S + S2-) concentration and the pH value of the water, using the formula :
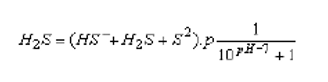
where p = activity coefficient depending on the ionic strength of water. For natural water it is about 0.92.
Hydrogen sulphide can be formed in decomposing rich organic mud, and escapes into the overlying water together with other gases (e.g. methane and carbon dioxide) formed by anaerobic degradation. In aerobic waters the H2S is rapidly oxidized
to sulphate; however, it is possible for fish living close to the surface of such muds to be exposed to hydrogen sulphide.
Carbon dioxide
Carbon dioxide is dissolved in water in its molecular gaseous state; only 10 % is in the form of carbonic acid H2CO3. These two forms of carbon dioxide together constitute what is termed free CO2. The ionic forms, i.e. fixed carbon dioxide, are represented by the bicarbonate and carbonate ions (HCO3– and CO32- respectively). Their presence is important for the buffering capacity of the water. The amounts of CO2 present in flowing surface waters are typically in the order of a few mg per litre, and seldom rise above 20 to 30 mg per litre. In stagnant surface waters the CO2 levels are stratified because of photosynthetic assimilation by phytoplankton, the upper strata usually having less free CO2 than the lower strata. If all the free CO2 in the surface strata is used for photosynthesis, the pH of the water there may rise above 8.3, and in waters of moderate bicarbonate alkalinity to 10.0 and above during the daylight hours. Ground waters from limestone or chalk strata usually contain several tens of mg of free CO2 per litre, and this may be important where well water is used for fish culture.
The toxic action of carbon dioxide is either direct or indirect. The indirect action of both free and bound CO2 is exerted on fish through its influence on water pH, especially where, as described earlier, the values rise to toxic levels. Also, changes in pH affect the toxicity of those chemicals which exist in the dissociated and nondissociated forms of which only one is toxic, such as H2S and ammonia.
A direct adverse effect occurs when there is an excess or absence of free CO2. In waters of low oxygen content, such as where intensive biodegradation is taking place, or where fish are kept or transported in a high density, or when poorly aerated ground waters are used, free CO2 may reach harmful levels. In such cases the diffusion of CO2 from the fish blood into the respiratory water is reduced, the blood CO2 rises and acidosis develops. If the rise in CO2 concentration is relatively slow (e.g. over 1 day), fish can adapt to the acidosis by increasing the bicarbonate concentration of the blood. Adapted fish can then suffer from alkalinosis if returned to water of low CO2 content.
In water of low O2 and high CO2, where gaseous exchange at the respiratory surface is limited, the fish increase their ventilation rate, become restless, lose equilibrium, and may die. Twenty mg free CO2 per litre is considered the maximum permissible concentration for trout (higher concentrations can cause kidney problems) and 25 mg free CO2 per litre is the maximum for carp (if the acid capacity is 0.5 mmol per litre at a pH of up to 4.5). The sensitivity of fish to free carbon dioxide declines with increasing acid capacity of water.
However, the more frequent occurrence is a lack of free carbon dioxide in water. Carbon dioxide deficiency occurs when too much free CO2 is utilized for photosynthetic activity by the phytoplankton, or when the water used in thermal power plants is artificially softened or when water is aerated more vigorously than necessary with CO2 free air. Free carbon dioxide concentrations below 1 mg per litre affect the acid-base balance in the fish blood and tissues, and cause alkalosis. A lack of free carbon dioxide is particularly harmful to cyprinid fry when they pass from endogenous to exogenous nutrition. Cyprinid fry respire through their body surface and are unable to regulate their acid-base balance by gill respiration. A low partial pressure of free CO2 in water is conductive to a high CO2 diffusion rate from the body, leading to alkalosis and finally to death. If the fry of cyprinids suffer from free CO2 deficiency, they gather close to the
water surface and show symptoms of suffocation even though the concentration of oxygen in the water is adequate (Taege, 1982).
A field study of toxic gill necrosis in carp, and preventative measures
Toxic gill necrosis was diagnosed in fish from the Dřemliny pond on May 2, 1984; about 50% of two-and three-year-old carp died (Svobodová et al., 1987, Fig. 5). The clinical signs of toxic gill necrosis in carp included the congregation of the fish in the deeper and shaded part of the pond and subsequently, in the advanced stage of disease the body surface darkened and there was a reduced or total absence of the escape response. Respiration was laboured and the fish did not feed.
Pronounced hyperaemia, oedematic swelling and increased accumulation of mucus in the gills are typical features of the patho-anatomic picture. These are followed by a gill necrosis and separation of the epithelium from the gill lamellae. The pillar cells of the gill lamellae are completely exposed over the whole lamellar surface. In the later stages of the disease, necrotic gill lamellae become detached and the margins of the gills are distorted. Histological and pathological examination reveals venostasis, swelling, vacuolization and separation of the respiratory epithelial cells from basal membrane in the gills. Associated with these effects is an increase in the activity of chloride cells in the lamellar epithelium. Dystrophic and necrobiotic cells from the respiratory epithelium (including chloride cells) create a compact mass of debris in the interlamellar space of gills. Extensive effects are characterized by a total lysis and necrotic changes in the cell nucleus. A significant increase in the ammonia level of blood serum in fish is a specific feature of these effects. The normal physiological level of ammonia in the blood serum of carp ranges from 350 to 800 μg N in 100 ml. The
ammonia level in the blood serum of carp with toxic gill necrosis fluctuates between 2000 and 4800 μg N per 100 ml, while 1000 to 2500 μg N in 100 ml serum are found in the early stages of toxic necrosis. In other gill diseases that cause necrosis, the following levels of ammonia in blood serum have been found (as N per 100 ml): bacterial diseases 420–900 μg, dactylogyrosis 500–730 μg, branchiomycosis 450–700 μg, and sphaerosporosis 450–960 μg.
The diagnosis of toxic necrosis is based on a detailed examination of fish. The main specific effect in carp is the elevated ammonia level in the blood serum. However, because such toxic gill necrosis can be caused by other unfavourable conditions in the pond environment (Fig. 5), a detailed hydrochemical and hydrobiological analysis of pond water is necessary to provide a definitive diagnosis.
Preventive measures to control frequent outbreaks of gill necrosis in carp in highly eutrophic ponds are centred on optimizing of the hydrobiological and hydrochemical conditions and ensuring the healthy state of fish stock (e.g. by a proper control of the feeding of fish). Stocking the ponds with fish at the correct time in the spring, and preventing or oxygen deficiency, are among the most important preventive measures.
In this context a simple biological test has been developed to determine the optimum timing for the spring stocking of two-year-old carp into ponds with a history of toxic gill necrosis. This test is based on the ability of carp to eliminate ammonia (under the existing physical and chemical conditions of the pond water) given as an oral dose of 350 mg. 100 ml-1 in starch gel. If the ammonia level in the blood serum decreases to the original value within 6 hours of the dose being given, the fish can be stocked in pond. On the other hand, if the ammonia level in the blood serum remains at a threefold higher level than the original value, the stocking of fish must be postponed until the physical and chemical conditions of pond water allow the fish to eliminate the toxic ammonia.
Application of the pesticide Soldep at a rate 200 ml ha-1 (depth of pond l m on average) can ensure the survival of the fish stock when an overproduction of zooplankton, followed by an oxygen deficiency, is expected. Soldep is effective in controlling the daphnid zooplankton and should be applied when there is still a reasonable phytoplankton community in the pond. Both during and after the Soldep application to the pond, standardized safety regulations must be followed (Svobodová and Faina, 1984).