DIAGNOSING THE CAUSE OF FISH POISONING
The diagnosis of fish poisoning is a difficult and complicated task because there may be a delay in the discovery of the mortality, and the fish and water are then not sampled at the time when the pollution occurred. In such cases the patho-anatomic changes in the fish are obscured by the onset of post-mortem changes and the toxic conditions that caused the fish to be poisoned may have been carried away from the affected area with the water flow or, in the case of natural events, reverted to normal. Hence, it is necessary to use all the available information and all possible and relevant analytical methods to detect the cause of the harm to fish and, where appropriate, to aquatic invertebrates.
The analytical study should begin with an assessment of previous records of factors that might influence natural changes, and of recent discharges that may have been made, and then performing the necessary physico-chemical and hydrobiological analyses of the water. If necessary, the bottom sediments, the periphytes and then the fish themselves should be examined. Bioassays to measure whether the water has an acute toxicity is an important tool in the diagnosis of fish poisoning.
Examination in situ
If the fish are observed to be behaving strangely or are dying, the following important actions should be taken at the site.
(a) Define the area within which the fish are seen to die or change their behaviour.
(b) Catch some of the affected or newly dead fish and submit them to veterinary examination as soon as possible. Recognized procedures should be used for their storage and/or preservation.
(c) Record the status of the zooplankton, phytoplankton and benthic communities.
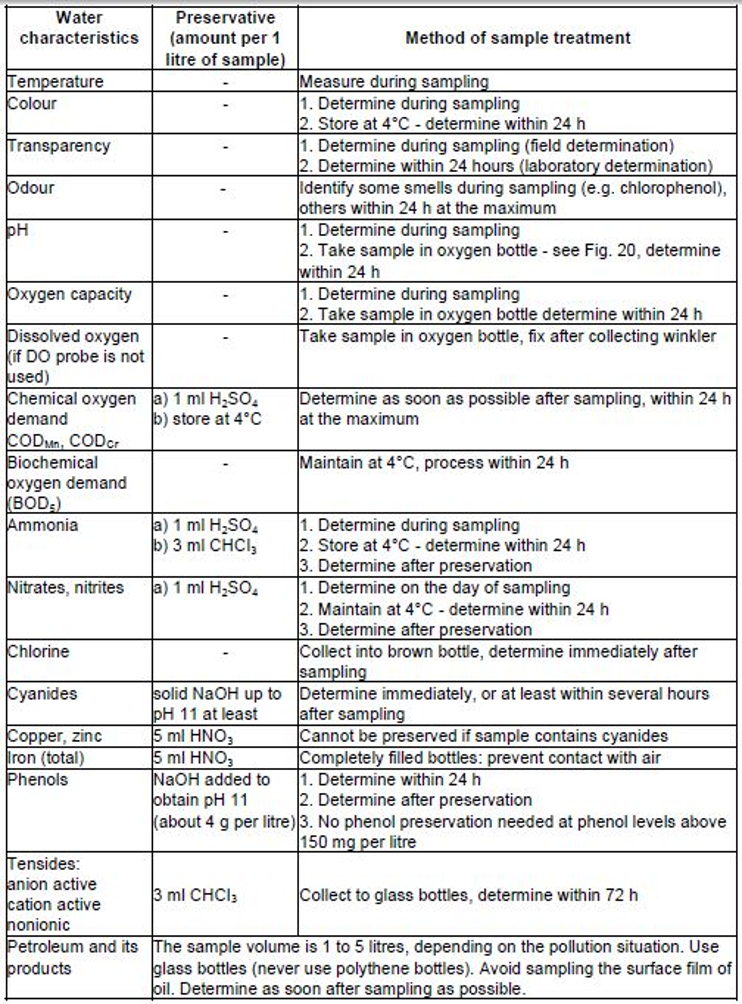
(d) Take water samples for hydrochemical analysis (some of the analyses and measurements must be carried out at the site e.g. O2, temperature, pH, transparency, smell, colour etc), and for the bioassay for acute toxicity.
(e) Draw a map of the affected area (and, if appropriate the surrounding areas) and record the site where water and sediment samples were taken. Fill in a form (an example is given below) for the in-situ examination.
If it is suspected that the fish might have died or changed their behaviour because of a discharge or use of a chemical in the vicinity of the affected water course or pond or lake, detailed information should be obtained of the time and method of the use and disposal, and of the identification and amount of the chemical applied.
If the fish are thought to have been killed by the use of a chemical in the vicinity of a water course or reservoir (e.g. a pesticide), the following data should also be included in the report of the site investigation:
- Day and hour when a crop was treated
- Chemical used (trade name, name and content of active ingredient, application rate, concentration)
- Method of application
- Identification at the land and type of crop treated, the area of the crop treated, distance from watercourse or reservoir, location of drainage ditches
- Name and address of the employer of the person who carried out the treatment
- Prevailing weather when the field was treated (wind direction, wind speed, rain, cloudiness) and rainfall records for the period between the application and the fish deaths.
- Map: A sketch map of the location, indicating the area where fish had died and the places where water samples were taken.